Alpha 1 4 Glycosidic Bond
12.six Disaccharides
- Folio ID
- 83190
Skills to Develop
- Identify the structures of sucrose, lactose, and maltose.
- Identify the monosaccharides that are needed to form sucrose, lactose, and maltose
- In Mueller's CHEM 109 course, you will non need to memorize the names or structures of the mono- or polysaccharides. Instead, you should be able to: (1) Identify the anomeric carbon; (2) Recognize a glycosidic bond and sympathise that water was removed from ii OH groups to form it; and (3) Characterization a glycosidic bond as alpha or beta.
- In Mueller's CHEM 109 class, the difference between reducing and non-reducing sugars is non covered.
Previously, you lot learned that monosaccharides tin can class circadian structures by the reaction of the carbonyl group with an OH group. These cyclic molecules can in turn react with another alcohol. Disaccharides (C12H22O11) are sugars equanimous of two monosaccharide units that are joined past a carbon–oxygen-carbon linkage known as a glycosidic linkage. This linkage is formed from the reaction of the anomeric carbon of i circadian monosaccharide with the OH group of a second monosaccharide.
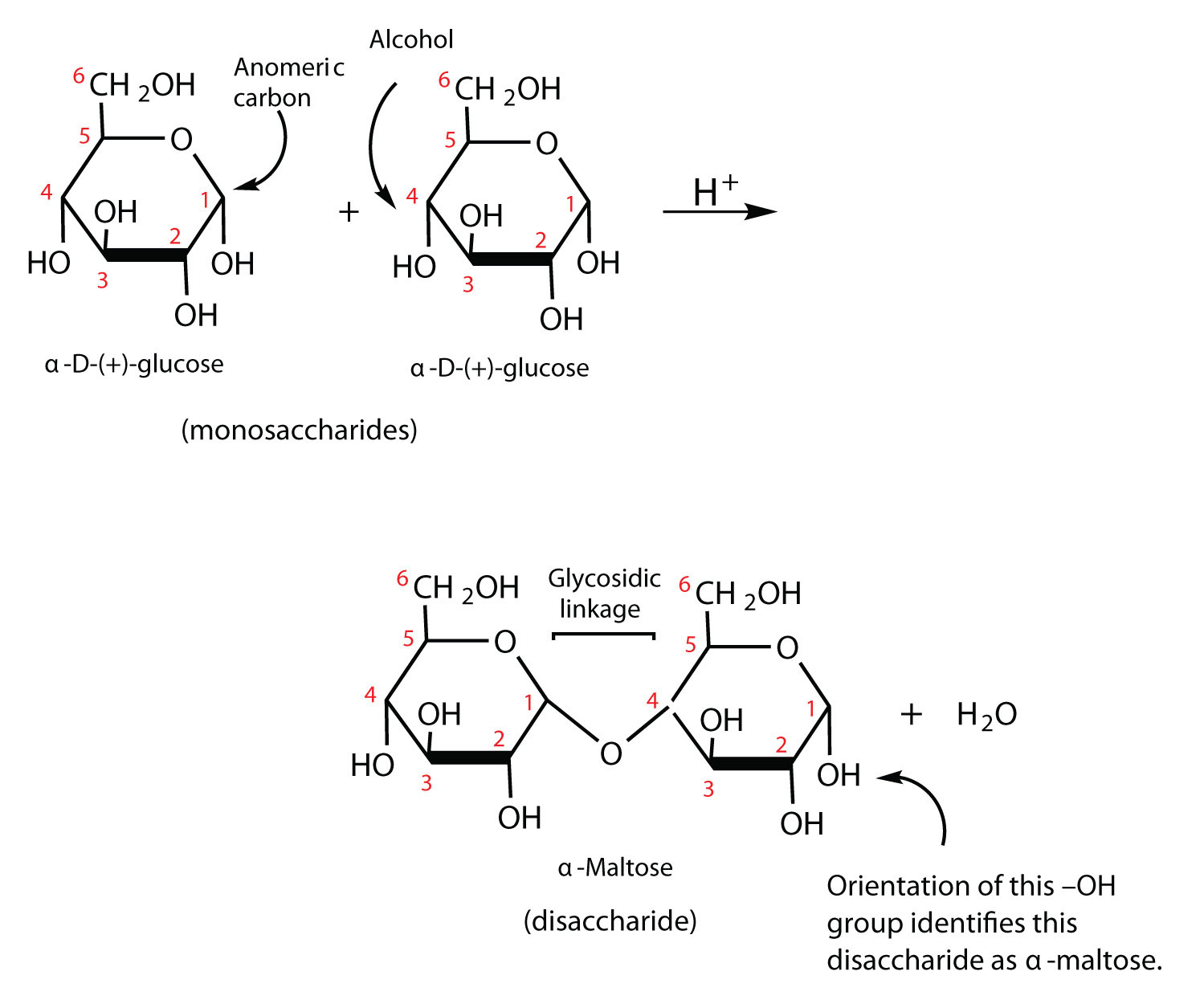
The disaccharides differ from one another in their monosaccharide constituents and in the specific type of glycosidic linkage connecting them. At that place are three common disaccharides: maltose, lactose, and sucrose. All three are white crystalline solids at room temperature and are soluble in water. We'll consider each saccharide in more detail.
Maltose
Maltose occurs to a limited extent in sprouting grain. Information technology is formed most often by the fractional hydrolysis of starch and glycogen. In the industry of beer, maltose is liberated past the action of malt (germinating barley) on starch; for this reason, information technology is oftentimes referred to as malt sugar. Maltose is about xxx% every bit sweet every bit sucrose. The human body is unable to metabolize maltose or whatsoever other disaccharide directly from the nutrition because the molecules are as well large to laissez passer through the cell membranes of the abdominal wall. Therefore, an ingested disaccharide must first be cleaved down by hydrolysis into its two constituent monosaccharide units. In the body, such hydrolysis reactions are catalyzed by enzymes such as maltase. The aforementioned reactions can be carried out in the laboratory with dilute acrid equally a catalyst, although in that case the rate is much slower, and high temperatures are required. Whether information technology occurs in the torso or a glass beaker, the hydrolysis of maltose produces 2 molecules of D-glucose.
\[\mathrm{maltose \xrightarrow{H^+\: or\: maltase} \textrm{2 D-glucose}} \tag{16.six.2}\]
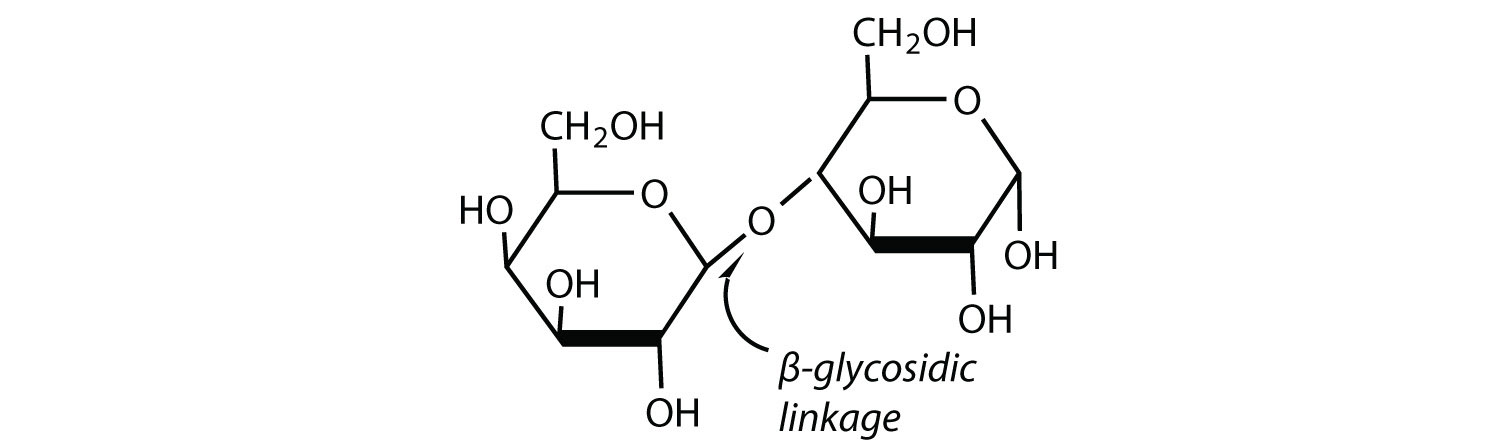
Maltose is a reducing sugar. Thus, its two glucose molecules must be linked in such a way equally to exit one anomeric carbon that can open to form an aldehyde group. The glucose units in maltose are joined in a caput-to-tail fashion through an α-linkage from the kickoff carbon cantlet of one glucose molecule to the fourth carbon cantlet of the second glucose molecule (that is, an α-1,iv-glycosidic linkage; meet Figure \(\PageIndex{1}\)). The bond from the anomeric carbon of the beginning monosaccharide unit is directed downward, which is why this is known as an α-glycosidic linkage. The OH group on the anomeric carbon of the second glucose can be in either the α or the β position, as shown in Figure \(\PageIndex{1}\).
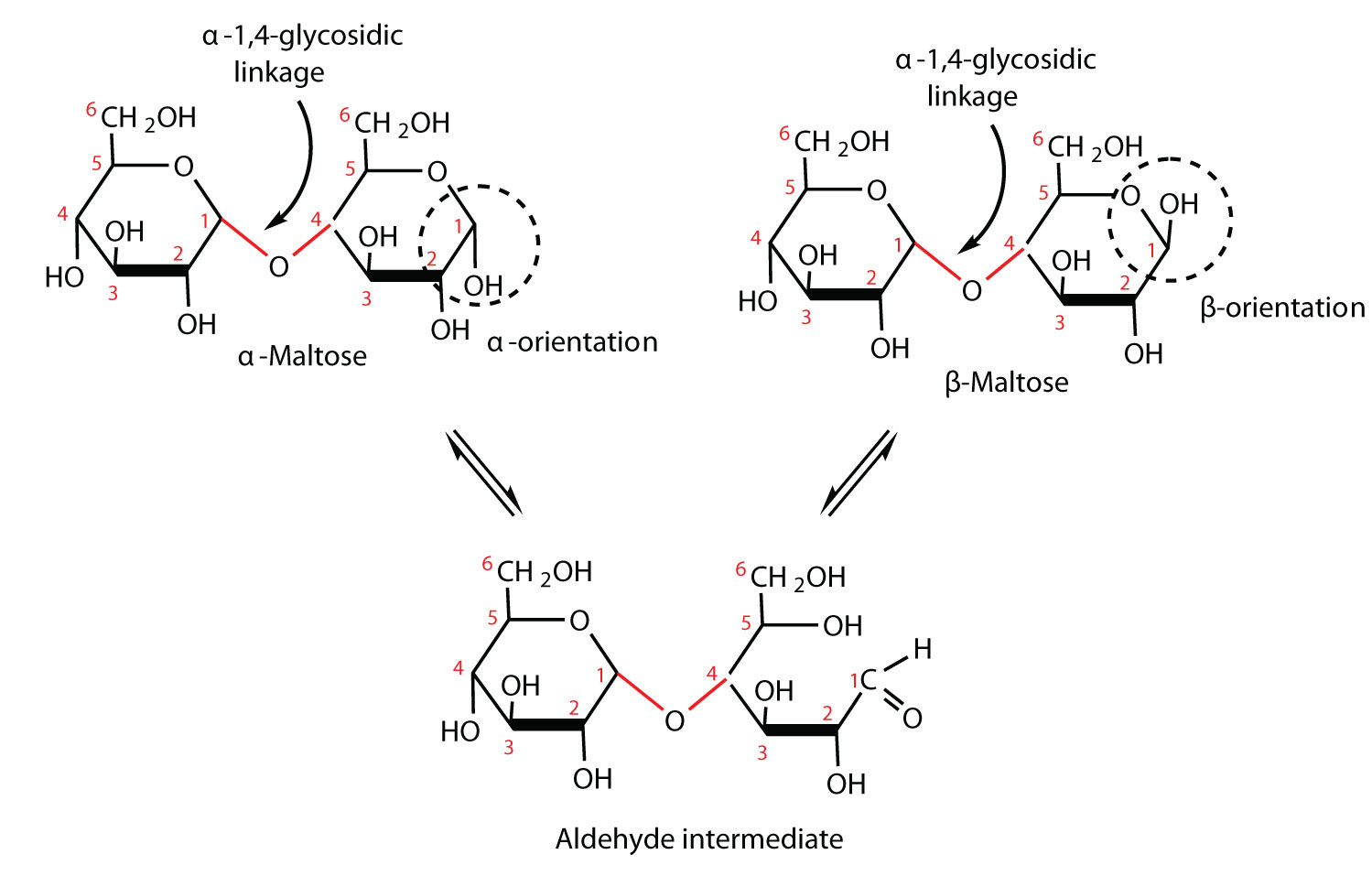
Figure \(\PageIndex{1}\) An Equilibrium Mixture of Maltose Isomers
Lactose
Lactose is known equally milk sugar because information technology occurs in the milk of humans, cows, and other mammals. In fact, the natural synthesis of lactose occurs only in mammary tissue, whereas most other carbohydrates are institute products. Homo milk contains virtually 7.5% lactose, and moo-cow's milk contains about 4.5%. This saccharide is one of the everyman ranking in terms of sweetness, existence about ane-sixth as sugariness every bit sucrose. Lactose is produced commercially from whey, a past-product in the industry of cheese. It is of import as an infant food and in the production of penicillin.
Lactose is a reducing sugar equanimous of ane molecule of D-galactose and one molecule of D-glucose joined by a β-ane,4-glycosidic bond (the bond from the anomeric carbon of the showtime monosaccharide unit being directed up). The ii monosaccharides are obtained from lactose by acrid hydrolysis or the catalytic activeness of the enzyme lactase:

Many adults and some children suffer from a deficiency of lactase. These individuals are said to be lactose intolerant considering they cannot digest the lactose constitute in milk. A more than serious problem is the genetic disease galactosemia, which results from the absenteeism of an enzyme needed to convert galactose to glucose. Certain bacteria can metabolize lactose, forming lactic acid as one of the products. This reaction is responsible for the "souring" of milk.
Instance \(\PageIndex{1}\)
example \(\PageIndex{ane}\):
For this trisaccharide, indicate whether each glycosidic linkage is α or β.
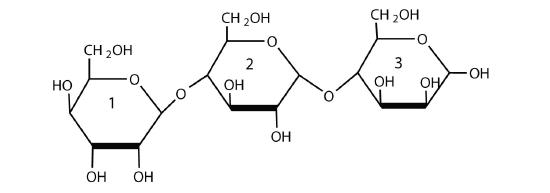
SOLUTION
The glycosidic linkage between sugars 1 and two is β because the bond is directed up from the anomeric carbon. The glycosidic linkage betwixt sugars 2 and 3 is α because the bond is directed downwards from the anomeric carbon.
To Your Health: Lactose Intolerance and Galactosemia
Lactose makes upwardly about 40% of an infant's nutrition during the first yr of life. Infants and pocket-size children have ane form of the enzyme lactase in their small intestines and tin can digest the sugar easily; however, adults ordinarily have a less agile form of the enzyme, and about seventy% of the world's adult population has some deficiency in its production. Every bit a result, many adults experience a reduction in the ability to hydrolyze lactose to galactose and glucose in their small intestine. For some people the disability to synthesize sufficient enzyme increases with age. Up to 20% of the US population suffers some degree of lactose intolerance.
In people with lactose intolerance, some of the unhydrolyzed lactose passes into the colon, where information technology tends to describe water from the interstitial fluid into the abdominal lumen by osmosis. At the aforementioned fourth dimension, abdominal bacteria may act on the lactose to produce organic acids and gases. The buildup of h2o and bacterial decay products leads to intestinal distention, cramps, and diarrhea, which are symptoms of the status.
The symptoms disappear if milk or other sources of lactose are excluded from the diet or consumed only sparingly. Alternatively, many food stores at present bear special brands of milk that have been pretreated with lactase to hydrolyze the lactose. Cooking or fermenting milk causes at least partial hydrolysis of the lactose, so some people with lactose intolerance are withal able to enjoy cheese, yogurt, or cooked foods containing milk. The most common handling for lactose intolerance, however, is the use of lactase preparations (e.g., Lactaid), which are bachelor in liquid and tablet form at drugstores and grocery stores. These are taken orally with dairy foods—or may be added to them directly—to assist in their digestion.
Galactosemia is a status in which 1 of the enzymes needed to convert galactose to glucose is missing. Consequently, the claret galactose level is markedly elevated, and galactose is institute in the urine. An baby with galactosemia experiences a lack of appetite, weight loss, diarrhea, and jaundice. The illness may result in impaired liver role, cataracts, mental retardation, and fifty-fifty decease. If galactosemia is recognized in early infancy, its effects can be prevented by the exclusion of milk and all other sources of galactose from the nutrition. Equally a child with galactosemia grows older, he or she usually develops an alternating pathway for metabolizing galactose, so the demand to restrict milk is not permanent. The incidence of galactosemia in the United States is 1 in every 65,000 newborn babies.
Sucrose
Sucrose, probably the largest-selling pure organic chemical compound in the world, is known as beet sugar, cane sugar, table carbohydrate, or merely sugar. Nearly of the sucrose sold commercially is obtained from carbohydrate cane and sugar beets (whose juices are xiv%–20% sucrose) by evaporation of the water and recrystallization. The dark brown liquid that remains after the recrystallization of sugar is sold as molasses.
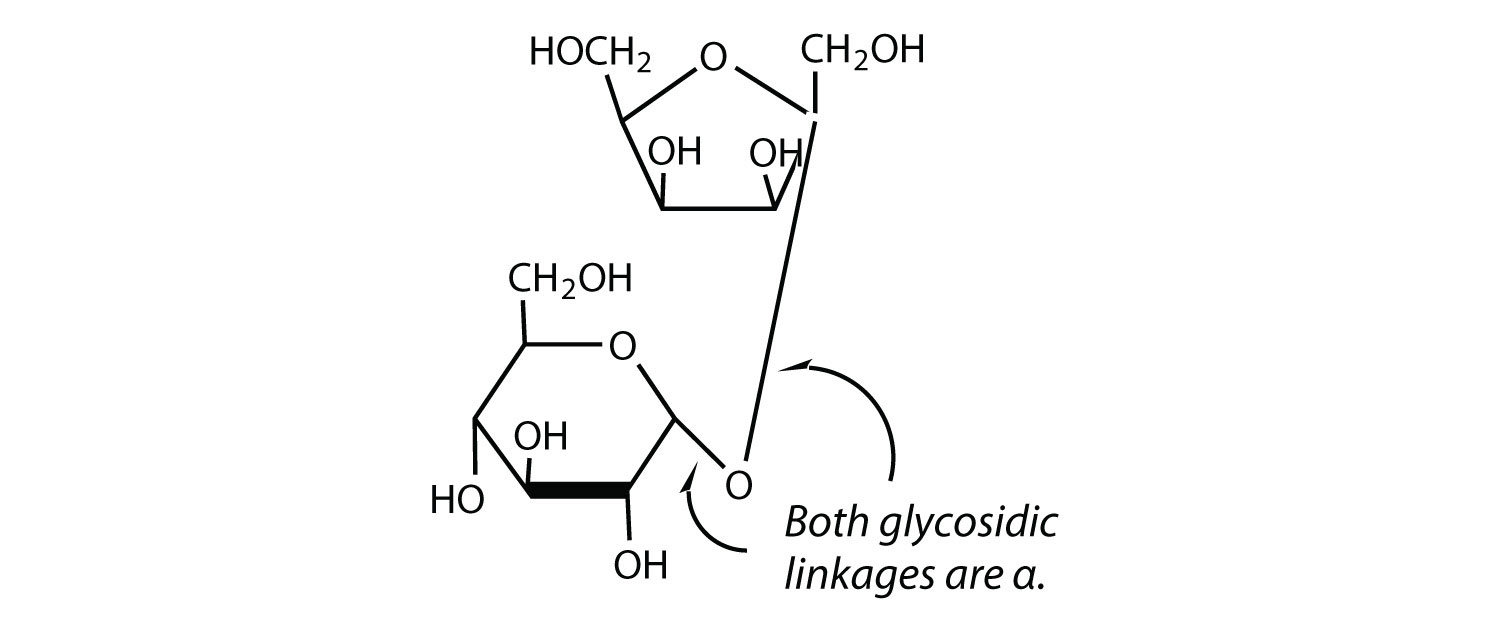
The sucrose molecule is unique among the mutual disaccharides in having an α-1,β-two-glycosidic (caput-to-caput) linkage. Considering this glycosidic linkage is formed by the OH group on the anomeric carbon of α-D-glucose and the OH group on the anomeric carbon of β-D-fructose, it ties up the anomeric carbons of both glucose and fructose.
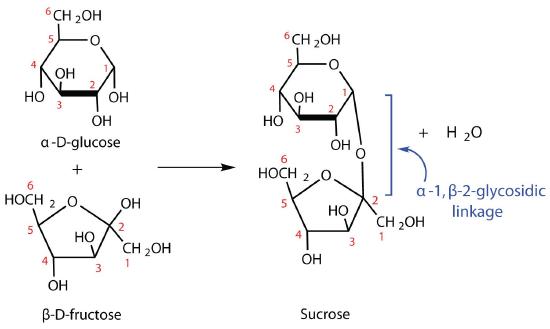
This linkage gives sucrose certain properties that are quite different from those of maltose and lactose. As long every bit the sucrose molecule remains intact, neither monosaccharide "uncyclizes" to form an open-chain structure. Thus, sucrose is incapable of mutarotation and exists in only one form both in the solid land and in solution. In addition, sucrose does not undergo reactions that are typical of aldehydes and ketones. Therefore, sucrose is a nonreducing sugar.
The hydrolysis of sucrose in dilute acid or through the activity of the enzyme sucrase (also known equally invertase) gives an equimolar mixture of glucose and fructose. This 1:ane mixture is referred to every bit capsize sugar considering information technology rotates airplane-polarized lite in the contrary direction than sucrose. The hydrolysis reaction has several practical applications. Sucrose readily recrystallizes from a solution, but invert sugar has a much greater tendency to remain in solution. In the manufacture of jelly and candy and in the canning of fruit, the recrystallization of sugar is undesirable. Therefore, weather leading to the hydrolysis of sucrose are employed in these processes. Moreover, considering fructose is sweeter than sucrose, the hydrolysis adds to the sweetening consequence. Bees carry out this reaction when they make honey.
The boilerplate American consumes more than 100 lb of sucrose every yr. About two-thirds of this amount is ingested in soft drinks, presweetened cereals, and other highly processed foods. The widespread use of sucrose is a contributing factor to obesity and tooth disuse. Carbohydrates such equally sucrose, are converted to fat when the caloric intake exceeds the body's requirements, and sucrose causes tooth decay by promoting the germination of plaque that sticks to teeth.
Summary
Maltose is composed of two molecules of glucose joined past an α-ane,iv-glycosidic linkage. Information technology is a reducing sugar that is found in sprouting grain. Lactose is equanimous of a molecule of galactose joined to a molecule of glucose by a β-1,4-glycosidic linkage. It is a reducing sugar that is found in milk. Sucrose is composed of a molecule of glucose joined to a molecule of fructose by an α-1,β-2-glycosidic linkage. It is a nonreducing sugar that is institute in sugar cane and carbohydrate beets.
Exercises
-
Point whether any glycosidic linkages are α or β.
a.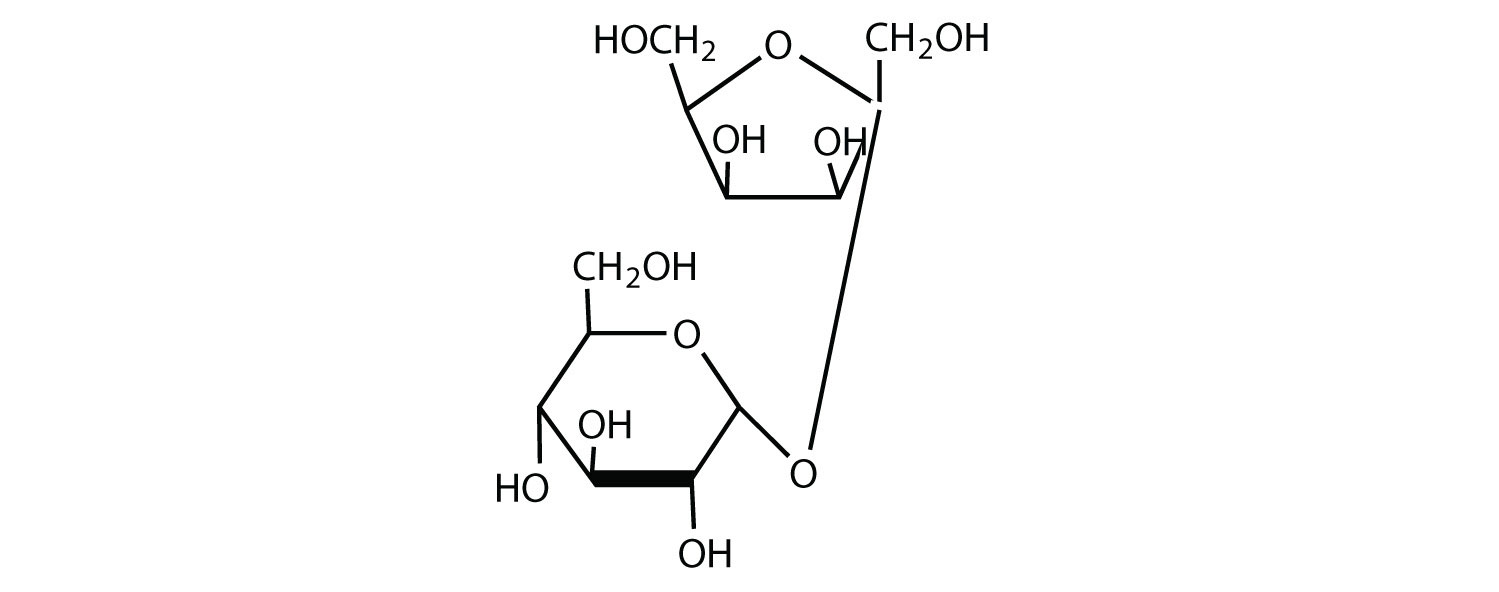
b.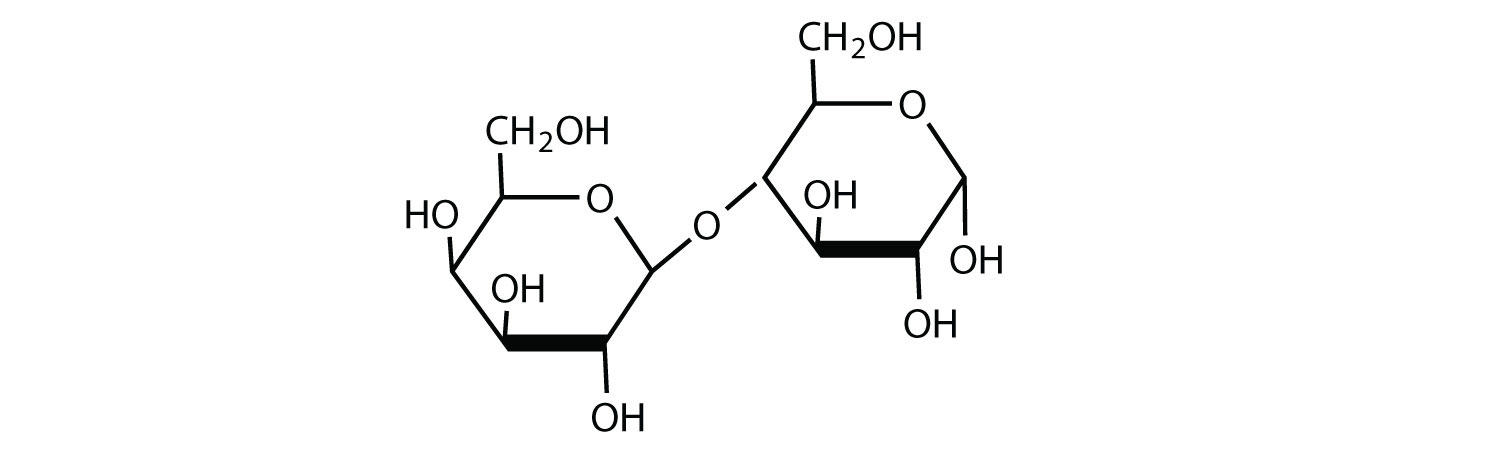
2. Melibiose is a disaccharide that occurs in some plant juices. Its structure is as follows:
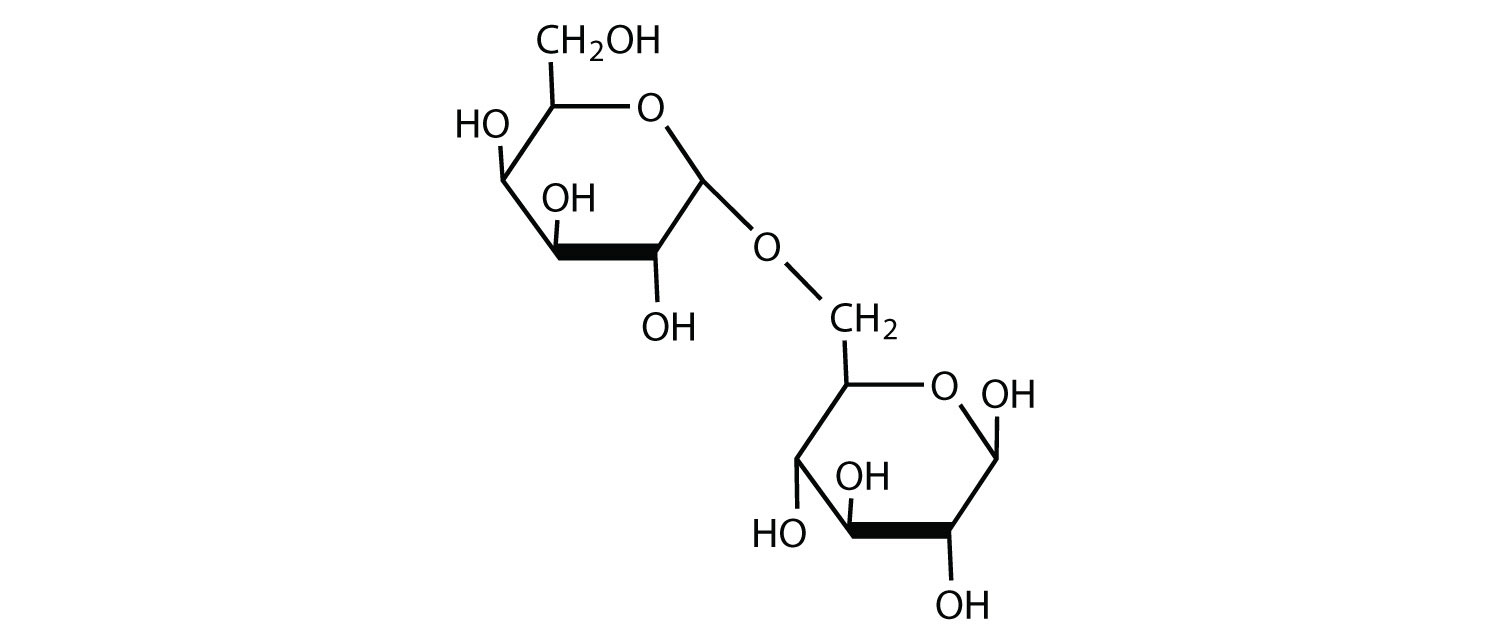
a. What blazon of linkage (α or β) joins the two monosaccharide units of melibiose?
b. Melibiose has a free anomeric carbon that is not involved in a glycosidic linkage. Circumvolve the anomeric carbon and indicate whether the OH group is α or β.
Answers
1.
2.
- α-glycosidic linkage
-
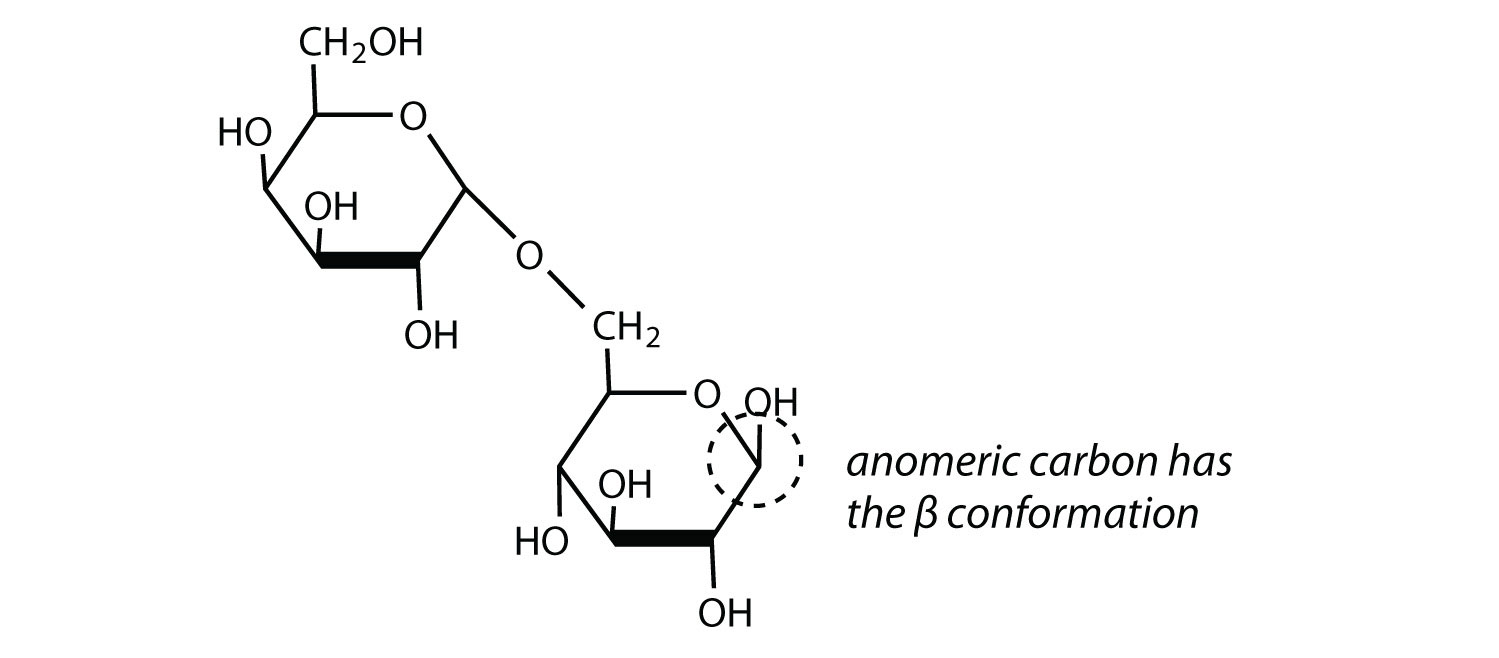
Alpha 1 4 Glycosidic Bond,
Source: https://chem.libretexts.org/Courses/University_of_South_Carolina__Upstate/USC_Upstate%3A_CHEM_U109_-_Chemistry_of_Living_Things_(Mueller)/12%3A_Carbohydrates/12.6_Disaccharides
Posted by: haylessairse.blogspot.com


0 Response to "Alpha 1 4 Glycosidic Bond"
Post a Comment